 |
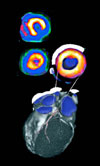
The isotope Strontium-82 is used for cardiac imaging via Positron Emission Tomography (PET) at hospitals nationwide. This is an image of a heart during a normal rest-stress myocardial perfusion PET study. More on Sr-82 production at LANL. |
Strontium
| Atomic Number: | 38 | Atomic Radius: | 249 pm (Van der Waals) |
| Atomic Symbol: | Sr | Melting Point: | 777 °C |
| Atomic Weight: | 87.62 | Boiling Point: | 1377 °C |
| Electron Configuration: | [Kr]5s2 | Oxidation States: | 2, 1[3] (a strongly basic oxide) |
History
Named after Strontian, a town in Scotland. Isolated by Davey by electrolysis in 1808, however, Adair Crawford recognized a new mineral (strontianite) as differing from other barium minerals in 1790.
Forms
Strontium is found chiefly as celestite and strontianite. The metal can be prepared by electrolysis of the fused chloride mixed with potassium chloride, or is made by reducing strontium oxide with aluminum in a vacuum at a temperature at which strontium distills off. Three allotropic forms of the metal exist, with transition points at 235 and 540°C.
Properties
Strontium is softer than calcium and decomposes in water more vigorously. It does not absorb nitrogen below 380°C. It should be kept under kerosene to prevent oxidation. Freshly cut strontium has a silvery appearance, but rapidly turns a yellowish color with the formation of the oxide. The finely divided metal ignites spontaneously in air. Volatile strontium salts impart a beautiful crimson color to flames, and these salts are used in pyrotechnics and in the production of flares. Natural strontium is a mixture of four stable isotopes.
Isotopes
Sixteen other unstable isotopes are known to exist. Of greatest importance is 90Sr with a half-life of 29 years. It is a product of nuclear fallout and presents a health problem. This isotope is one of the best long-lived high-energy beta emitters known, and is used in SNAP (Systems for Nuclear Auxilliary Power) devices. These devices hold promise for use in space vehicles, remote weather stations, navigational buoys, etc., and where a lightweight, long-lived, nuclear-electric power source is needed.
Uses
In addition to the medical imaging application described in the image caption above, strontium has found use in producing ferrite magnets and in refining zinc. Strontium titanate is an interesting optical material as it has an extremely high refractive index and an optical dispersion greater than that of diamond. It has been used as a gemstone, but is very soft. It does not occur naturally.
